Primary.Health’s cutting-edge solutions for H5N1 testing and surveillance, in collaboration with public health agencies, represent a major step forward in the global response to this emerging threat.
The emergence of highly pathogenic avian influenza (HPAI, bird flu) H5N1 has triggered a global public health concern, demanding swift and effective interventions to prevent its spread [1-4]. As a leading healthcare technology company, Primary.Health is at the forefront of developing innovative solutions to support public health agencies in responding to this evolving threat. By leveraging cutting-edge technology, strategic partnerships, and novel workflows, Primary.Health is revolutionizing H5N1 testing and surveillance, empowering public health authorities to make data-driven decisions and implement targeted interventions.
Provide a testing solution to dairy and poultry farms, in collaboration with state or public health organizations.
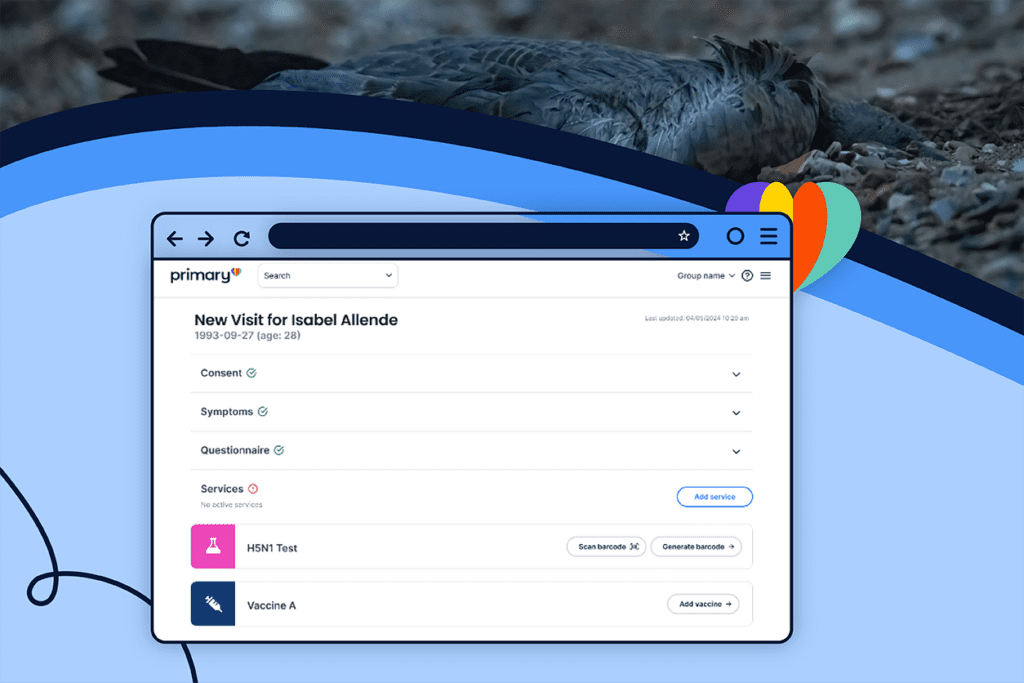
One of Primary.Health’s key initiatives is to provide a comprehensive testing solution for dairy and poultry farms, which are particularly vulnerable to H5N1 outbreaks. In collaboration with state and public health organizations, Primary.Health has developed a workflow that streamlines the testing process, ensuring rapid detection and response to potential H5N1 cases.
Obtain a CLIA-Waiver through the Primary medical group
The solution begins with obtaining a CLIA-Waiver through the Primary medical group, enabling the deployment of rapid lateral flow and molecular CLIA-Waived testing kits to dairy and poultry farms. By setting up testing groups with customized registration, consent, and data access permissions, Primary ensures that sensitive health information is protected while allowing for the prompt reporting of positive Flu A samples to state public health databases.
Local Public Health Labs or overflow commercial labs for lab-based confirmation and sequencing
To confirm and characterize positive Influenza A cases, Primary has established a seamless integration with public health laboratories (PHLs) and overflow commercial labs. This collaboration allows for lab-based confirmation and sequencing of positive samples, providing public health authorities with the critical information to track the spread of H5N1 and develop targeted interventions.
Primary’s role in this initiative is multifaceted, showcasing the company’s commitment to leveraging technology for public health [1-4]. By providing a robust software platform for managing custom workflows, lab orders, devices, and results, Primary ensures that the testing process is efficient, accurate, and user-friendly. The company’s provider group takes responsibility for ordering tests and following up on cases, addressing the unique challenges agricultural workers who lack access to health insurance may face.
Moreover, Primary’s expertise in interoperability enables seamless data exchange between testing sites, public health labs, commercial labs, and in-field rapid tests. This integration is crucial for real-time data analysis and outbreak detection, empowering public health authorities to respond quickly and effectively to emerging H5N1 threats.
In addition, Primary offers advanced data analytics and dashboarding capabilities for epidemiologists and business leaders, providing powerful tools for epidemiological investigative work. By visualizing and analyzing H5N1 testing data, health experts can identify trends, map the spread of the virus, and develop evidence-based strategies for containment and prevention.
Public health labs, “PHLs,” are critical in this collaborative effort, bringing their expertise and resources to the fight against H5N1 [1-4]. PHLs are the backbone of the confirmation and sequencing process, ensuring the accuracy and reliability of H5N1 testing results. Public health staff also contribute to the on-the-ground implementation of the testing solution, providing guidance and support to dairy and poultry farms.
Furthermore, public health agencies provide the necessary funding, oversight, and epidemiological prioritization to ensure the success of the H5N1 testing initiative. Public health authorities can develop and implement effective policies and strategies to combat the spread of H5N1 by working closely with Primary and other stakeholders.
Primary.Health’s cutting-edge solutions for H5N1 testing and surveillance, in collaboration with public health agencies, represent a major step forward in the global response to this emerging threat. By harnessing the power of technology, data analytics, and cross-sector public-private partnerships, Primary empowers public health authorities to make informed decisions, implement targeted interventions, and save lives. As the initiative progresses, it will serve as a model for effective, data-driven public health responses to infectious disease outbreaks, showcasing the immense potential of innovative technology in addressing the complex challenges of the 21st century.
Bibliography
- U.S. Food and Drug Administration. (2024). Updates on Highly Pathogenic Avian Influenza (HPAI). Retrieved from https://www.fda.gov/food/alerts-advisories-safety-information/updates-highly-pathogenic-avian-influenza-hpai
- World Health Organization. (2021). Avian Influenza Weekly Update Number 825. Retrieved from https://www.who.int/docs/default-source/wpro—documents/emergency/surveillance/avian-influenza/ai-20211203.pdf
- Nguyen, T. H., Nguyen, T. T., Nguyen, T. T., Nguyen, T. M., & Le, T. Q. (2021). Highly Pathogenic Avian Influenza A(H5N1) Viruses: Recent Developments and Potential Threats. Viruses, 13(7), 1292. https://doi.org/10.3390/v13071292
- Yang, L., Zhu, W., Li, X., Chen, M., Wu, J., Yu, P., Qi, S., Huang, Y., Shi, W., Dong, J., Zhao, X., Huang, W., Li, Z., Zeng, X., Bo, H., Chen, T., Chen, W., Liu, J., Zhang, Y., … Wang, D. (2017). Genesis and Spread of Newly Emerged Highly Pathogenic H7N9 Avian Viruses in Mainland China. Journal of Virology, 91(23). https://doi.org/10.1128/JVI.01277-17
